Release Notes 2.7
Released August 4th 2021
Improved Article System • Quality & Test-Management • Goods Management • Plant Inventory
Article system
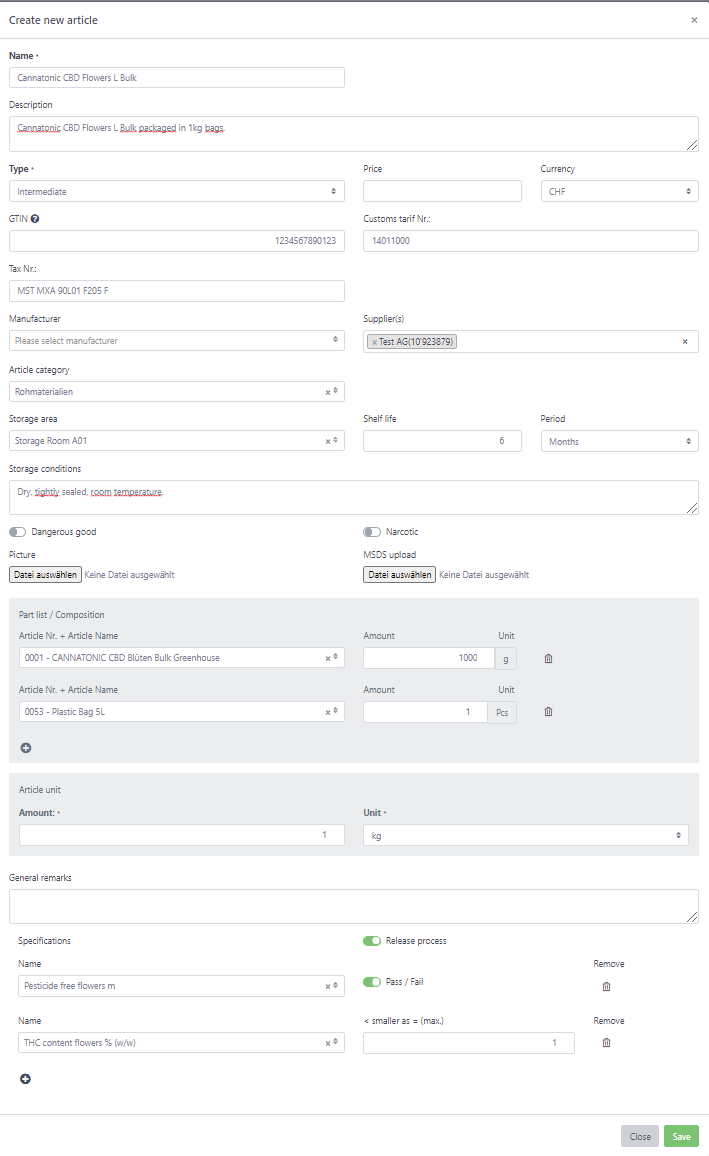
Create and manage the articles you produce and that you use in your company. We have built an extensive article system that allows you define precisely what products you are using and what quality criterias must be met.
Set basic properties such as Name, Description and Price.
Upload a picture of the article.
Select from a preset of article types (Raw material, intermediate, final product, additive, seed, plant) to help in organizing the materials you use and manufacture.
Add additional article identifiers like Customs tarif Nr. for easier export as well as Tax Nr.
Specify who can supply this article to you and who manufactures it to speed up the troubleshooting in case of a non-conformity
Set the Shelf life, storage conditions as well as the preferred storage area to make sure articles are properly stored.
Identify if the article is a dangerous goods or narcotic to make sure necessary precautions are met.
Upload the material safety data sheet (MSDS)
If the article is manufactured, define how it is composed.
Define if you want to use our release system to make sure the article is fullfilling your quality specifications. Check the testing management on how to set up your testing methods.
Article categories
Manage your article by creating your own categories. You can create root categories and any amount of sub categories in a tree structure . We have prepared the system to be able to import category structures for down the line inclusion of GS1 categorization.
Quality control
Meet our new quality control feature that will aid you in manufacturing and using materials that are according to your specifications. While articles contain all the general information, article batches contain all information of the specific batch of that article. When it was manufactured, shelf life, manufacturing protocols, analysis reports, certificate of analysis, what batches have been used to manufacture it and more. All relevant information is just one click away.
Release your products in a simple 3-step process:
Testing: analyze the batch, use our integrated sample management for easy coordination
Approval: a technician checks the results and approves if the manufactured batch is according to specification
Release: the responsible person (qualified person) can release the batch making it available for goods out or further production.
The release needs approval from atleast 2 users, it is impossible for one user to release a batch. This minimizes the risk of oversights.
Batches can be blocked if they are not within specifications.
The batch code in our system uses 7 digits and capital letters without 0 and 1 to avoid mix ups with O and I. You are free to use your own batch coding system without any problems.
Once a batch is released, the certificate of analysis is automatically generated by the software as a pdf file.
Goods in
The guided goods in system allows you to perfrom a goods in process to digitalize your stock. With the implemented quality control you can check and release new batches coming in from suppliers to make sure you are always receiving raw materials and products that are within your quality specifications.
Guided goods in process to make sure all necessary documentation is available
Document if packaging is damaged
Make sure labels are attached correctly.
Take and save pictures
Add notes
If the incoming good has specifications, the quality has to be checked and released using our quality control feature before it can be used in manufacturing processes.
Goods out
Document who is receiving which products and from which batch. The system autmatically creates a delivery note and gives you quick access to relevant data such as certificate of analysis of the shipped batches.
Testing management
How do you take your samples, with which methods are you testing and what tests do you perform do guarantee the quality of your product? With this new feature you can clearly define how samples have to be taken, what methods are used to examine them and what exactly is tested.
Sample collection SOP: create any amount of SOPs or collect them all in one master SOP that defines how different materials are sampled.
Testing method: By uploading an SOP you can define how something has to be tested and by selecting equipments you can go one step further and define what equipments have to be used to test samples.
Tests: define what the result of a test is going to be.
This allows you to attribute tests to your articles and specify what results have to be met to satisfy your quality specifications.
Plant inventory
In the plant inventory you have a clear overview how many plants are in your cultivation, sorted by what phase and batch they are in. Allowing you to manage your clones with an ancestry of each individual plant. Adding plants to your cultivation is more straight forward and you always know how many are still available.
Overview and ancestry of every plant
Management of clones, taking clones and adding them to a new batch or start a new batch from scratch.
Samples
Managing your samples has been simplified. The previous packages system has been replaced with the new sample system.
Take samples from plant, harvest, drying and article batches.
Automated Sample ID and label
Overview of ongoing and finished testing
CBD Test integration
Automated analysis assignment